Child participation in research
The information reflects the situation up to 1 January 2014. Updates are made based on subsequent developments as soon as FRA is aware of a change.
If you have any feedback on the data we would be happy to receive your comments by email at: childrights@fra.europa.eu.
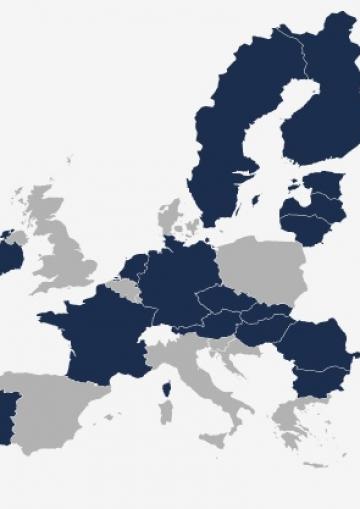
The map below shows the age requirements for parental consent for children's participation in research in each EU Member State.
Requirements for parental consent
Source: FRA, 2014
| Parental consent is always required for children up to 18 years old | |
| Regulation on parental consent varies depending on contexts, and parental consent is required for children up to 18 years old in school settings | |
| Parental consent is required for children under the age of 15 or 16 | |
| Parental consent is required for children under the age of 14 | |
| There is no clear regulation about parental consent and age groups |
The information provided below describes legal requirements and procedures for involving children in research, including in particular procedures of ethics approval and informed consent of children and their parents for all EU Member States.
| Country | Key points | Conclusions |
|---|---|---|
| Austria |
Context of legal framework and ethical codes of conduct: Data Protection Act 2000[1] Age: n/a Consent: In social research - Children might participate in surveys provided that the child’s parent has given his/her consent[2] Role of children: n/a Role of parents: n/a Schools: n/aInstitutions: n/a Ethical approval: n/a |
|
| Belgium |
Context of legal framework and ethical codes of conduct: Law of 8 December 1992 on the protection of privacy in relation to the processing of personal data [7] Age: n/a Consent: n/a Role of children: n/a Role of parents: n/a Schools: When parents are not involved in the carrying out of the research, the director, vice-director or teacher will provide the consent in place of the parents. This consent will also not always be given in a written form, as there is no general consensus on how consent should be given [8] Institutions: n/a Ethical approval: n/a Other sources: [9] |
|
| Bulgaria |
Context of legal framework and ethical codes of conduct: Child Protection Act [10]; Ethical Code for the People Working with Children [11]; Ethical Code of the Bulgarian Media [12] ; Protection of Personal Data Act, art 5 [13] Age: n/a Consent: Non-Obligatory: researchers have to inform the family and involve children in the decision-making process. Every child has the right to freedom of expression of opinion and the right to formulate own views and to freely express them [11] Role of children: When the child is over 14, his/her consent is needed [10] Role of parents: Current practice - Parental consent is always needed [14] Schools: n/a Institutions: Social Assistance Department (which is the body that officially applies measures applied to children at risk by the Child Protection Departments subordinated to the Social Assistance Departments) should also give written opinion if the research includes collection and dissemination of personal data Ethical approval: n/a Other Sources: [15] |
|
| Croatia |
Context of legal framework and ethical codes of conduct: Code of Ethics for Research Involving Children [16] ; National Pedagogical Standard for Preschool Education [17], National Pedagogical Standard for Elementary Education [18], National Pedagogical Standard for Secondary Education [19]; Act on the Protection of Patients’ Rights [20] Age: < 14 parental consent > 14, child consent [16] Consent: Informed and obligatory Parental consent for <14 children >14 children consent oral or written Duty to inform the participants of the results of the research Role of children: Children between 7 and 14: have to be informed about the research in terms appropriate to their age Role of parents: Parents or guardians have to be informed about the research even when parental consent is not required Schools: Written consent from the parents of each child involved in the research Procedure length: between three to six weeks [17] [18] [19] Institutions: n/a Ethical approval: Approval needed from the relevant institutional ethics committee [16] Other Sources: [21] |
|
| Cyprus |
Context of legal framework and ethical codes of conduct: Operational guidelines of the Cyprus National Bioethics Committee (CNBC) [22]; Centre of Educational Research and Evaluation (CERE), Guidelines for the submission of the online application [23] Age: n/a Consent: Practice - Parental written consent form and child's opinion should be respected [24] Role of children: Practice - Child opinion should be respected Role of parents: n/a Schools: Practice - Written consent is required from the student's parents, when a study is conducted in public schools. Obligatory approval is needed from the Directorates of the Ministry of Education and Culture (MoEC) through the Center of Educational Research and Evaluation (CERE). The whole approval procedure may take four to five weeks. After the completion of the research study, the researcher has to submit a summary of the results to the MoEC. [23] [25] Institutions: Approval needed from the Director of Social Welfare Services (replacing parental consent) [26] Ethical approval: n/a |
|
| Czech Republic |
Context of legal framework and ethical codes of conduct: Ethical Framework of Research by Ministry of Education [27] , Youth and Sports Esomar guidelines [28] ; Ethical Regulations of Czech Association for Social Anthropology; Psychology, Sociology, Pedagogy Age: Informed consent by parents for <14 [27] [28] Consent: Non-obligatory. No regulation on the need of written informed consent but written form is preferred [27] Role of children: Child’s expression of its voluntary participation. Non-obligatory Role of parents: Informed consent by parents/guardians. Non-obligatory Schools: <14: Informed consent from teacher or schoolmasters. Non-obligatory for pre-school age: presence of parents or other person close to child [28] Institutions: n/a Ethical approval: n/a |
|
| Denmark |
Context of legal framework and ethical codes of conduct: Act on Processing of Personal Data [35] ; Guidelines of the Danish Social Science Research Council (DSSRC) [36] ; NCHRE [37] Age: n/a Consent: Non-Obligatory Role of children: The child must receive information orally Role of parents: The parents must receive sufficient information Schools: n/a Institutions: n/a Ethical approval: n/a Other Sources: [38] |
|
| Estonia |
Context of legal framework and ethical codes of conduct: Personal Data Protection Act; [39] Code on Market and Social Research (ICC/ESOMAR) [40] Age: n/a Consent: Non-obligatory. The consent of the parent shall first be obtained before interviewing children. Oral consent is enough [40] Role of children: n/a Role of parents: n/a Schools: The teacher can consent. Oral consent is enough (Non-obligatory) [40] Institutions: n/a Ethical approval: In absence of ethic committee for social research, some researchers submit applications to the medical ethics committee (Tallinn Medical Research Ethics Committee Research Ethics Committee of the University of Tartu [42] and the Tallinn Medical Research Ethics Committee [43]). The following information needs to be provided to the Commitee in Tartu: description of the research methodology that includes questionnaires and tests; an analysis of ethical aspects of the research; the information sheets for the informants and the forms of consent; list of researchers carrying out the research, their academic qualifications; information about the financing of the research [42] Other Sources: [41] |
|
| Finland |
Context of legal framework and ethical codes of conduct: Personal data Act (1999) [44] ; TENK-Recommendations from the National Advisory board on research ethics-TENK [45] ; Child Custody and Right of Access Act (362/1984) [46] Age: n/a Consent: No specific provision for children. Recommendations from the National Advisory board on research ethics-TENK [44] [45] Role of children: Researchers must always respect a children's autonomy and the principle of voluntary participation Role of parents: No parental consent required if research is pre-school or school, no individual identification information is collected, and school principal thinks research is useful for institutions. When consent is not sought from parents, and children under 15, an ethical pre-evaluation should be conducted. Schools: Practice - May differ from municipality to municipality, but usually approval from local school board is necessary. Institutions: In institutional settings such as prisons, child protection institutions, hospitals, homes for the elderly and similar places, it is recommended that consent is sought from each and every subject [45] Ethical approval: Not required for social research, but increasingly used in the fields of nursing, psychology and physical education. The ethical approval is sought through regional ethics committees. Universities and other institutions can also have their own ethical approval[45] Other Sources: n/a |
|
| France |
Context of legal framework and ethical codes of conduct: French Data Protection Authority (CNIL) 'Education Guide' and the 'Higher Education and Research Guide’ [48] ; France, Public Health Code (Code de la santé publique), article L1122-1-1 [49] ; Code of Education (Code de l’éducation) [50] Age: n/a Consent: n/a Role of children: n/a Role of parents: n/a Schools: When research is undertaken in both state and private schools, the consent of the parents or the children is never expected. However, French law requires that information on the research being conducted is provided to the families, which in turn allows them, if they wish, not to send their children to an establishment undertaking research. Students' right to prior information must be guaranteed. The survey questionnaire sent to students should mention the wording of Law No. 78-17 of 6 January 1978 relating to data protection and civil liberties [50] Institutions: n/a Ethical approval: n/a |
|
| Germany |
Context of legal framework and ethical codes of conduct: Association of Market and Social Research Agencies - ADM guidelines Age: n/a Consent: Depends on Federal States and context Role of children: n/a Role of parents: n/a Schools: In some States minors up to age of 18 written informed consent by parents Institutions: Research in juveniles detention centres (14-24); approval by responsible ministers of justice. Situation comparable to school settings in regard to federal responsibilities Ethical approval: Ethical codes of conduct by scholarly associations (Sociology, Psychology, Education), but no remarks on research involving children Other Sources: [55] |
|
| Greece |
Context of legal framework and ethical codes of conduct: Civil Code [56] ; Personal Data Law [57] ; Hellenic Data protection authority and self-regulatory code of advertisement & communication [58] ; Code of media Ethics; Law 2619/1998 [59] , Law 3984/2011 [60] Age: Different degrees of responsibility from 10, 14 and 15 years [56] Consent: n/a Role of children: Obligatory - Child consent required in school research Role of parents: Parental consent is required when research in school, but no specification of age groups. Parental consent is required always for children with disabilities. Schools: There is a special form on written consent for parents and children in the website of the Hellenic School Network. Institutions: Researchers apply to institutions, who forward to Ministry of Health or Ministry of Justice. If personal data are necessary, then also approval by Data Protection authority is necessary [62] Ethical approval: n/a Other Sources: n/a |
|
| Hungary |
Context of legal framework and ethical codes of conduct: Act on informational self-determination and freedom of information [63] ; Codes of ethics of different Universities (psychology departments) [64] Age: n/a Consent: Obligatory parental consent is required for children <16, if processing of personal data. Consent of children >16 is sufficient [63] Role of children: Oral consent if under 14 and written consent over 14 [64][65] Role of parents: Parental consent for children <16 Schools: n/a Institutions: n/a Ethical approval: Different practices according to University, usually all projects including children require ethical approval [64] [65] [66] Other Sources: n/a |
|
| Ireland |
Context of legal framework and ethical codes of conduct: Data protection acts 1988 and 2003 [67] ; Ethical review and children’s research in Ireland, Office of the Minister for Children and youth affairs (2010) [68] ; Statutory instruments N 190, 878 and 374 [69] Age: Data protection acts with no specific reference to children [67] Consent: No standardised regulations Role of children: The review requires further involvement of children. The current practice gives a more prominent role to parents than to children [68] Role of parents: Practice seems to require parental consent for children up to 18. For adolescents the need for parental consent depends from each individual ethical committee Schools: n/a Institutions: n/a Ethical approval: Usually a Research Ethics Committee is formed for the specific project. There are many local or issue-specific Committees in different institutions and universities. No national coordination/overseeing Other Sources: n/a |
|
| Italy |
Context of legal framework and ethical codes of conduct: Civil Code (1942)[70], Personal data Act (2003)[71], different deontological codes published by the National Data Protection Authority [177] and by professional associations Age: There are several codes of ethics of professional associations
Role of children: If child ability to understand the request, then also informed consent from child is necessary (Non-Obligatory) (ibidem) Role of parents: Informed consent of parents. Non-obligatory (ibidem) Schools: In addition to the info above, also informed consent of headperson or principal of school: Non-obligatory (ibidem) Institutions: n/a Ethical approval: n/a |
|
| Latvia |
Context of legal framework and ethical codes of conduct: Protection of the rights of the Child Law [72]; Personal Data protection law [73] ; Code of the Latvian sociological association [74] ; Institute of Sociological Research [75] ; Code of the Latvian sociological association [76] Age: n/a Consent: If personal data processing, no specifics on children, general consent of data subject. Role of children: Informed consent of all research subjects, including of children below 16 years to the extent they are able to give it [74] Role of parents: Parental consent required for children <16, unless 1) research imposes a minimum risk to the child, or 2) the informed consent of parents is not a reasonable requirement (e.g. abused children, use of drugs) Schools: Practice - Consent by school director and children. In research with children of 7 years, also parental consent [75] Institutions: Approval of authorities and children Ethical approval: No approval, but possibility to complain about sociologist to the Sociological Association [74] Other Sources: [77] |
|
| Lithuania |
Context of legal framework and ethical codes of conduct: Law on the Legal protection of Personal Data [78] ; Civil Code [79] Age: Civil Code- Children between 14 and 18 certain capacity to act Consent: Practice: Written consent for children <16 [80] Role of children: Practice: Children consent >16 Role of parents: Written consent for children <16 Schools: Practice - Approval from school administration or head teachers. If research does not cover sensitive data (as above) parental consent is often not required for children >16 Institutions: Example of research on sexual abuse in residential institutions: informed consent of administration of residential institutions (i.e. legal guardians)[83] Ethical approval: n/a |
|
| Luxembourg |
Context of legal framework and Ethical codes of conduct: Civil Code [88] , NCDP (National Commission for Data Protection) [86], NCER (Comité National d’Ethique et de Recherche) [90] Age: Informed consent by parents for <14 [86] Consent: Non-obligatory Signed consent from parents is recommended as best practice. For all children <12, recommended <14 by parents and children Role of children: for children between 12 and 14 consent of children plus parental consent is recommended. Non-obligatory Role of parents: Informed consent for children <14. Non-obligatory, recommended Schools: Information to be provided - objective, contribution, anonymity, free participation, questionnaire for parents, stamped envelope [87] Institutions: n/a Ethical approval: Obligatory ethical approval of research by national body if personal data is used. No specific information on children and age groups. Guidelines highlight ethical issues particularly addressing children and informed consent [86] [89] |
|
| Malta |
Context of legal framework and ethical codes of conduct: Data Protection Act [93] ; Criminal Code; Civil Code [94] Age: 18, where parents jointly represent their children [94] Consent: Parent till up to the age of 12; >12 legal representative additionally consent by children should be considered. [95] When processing personal data the consent of the parents is required for children up to 18 years. Role of children: n/a Role of parents: n/a Schools: Ethical Approval is obligatory in schools. Written consent by parents, consult with Head of Schools, right to object any request. [97] Institutions: Children under care - Ethical approval by children and young persons’ advisory board, then from child participating [98] Ethical approval: Non-Obligatory - By UREC: 30 days |
|
| Netherlands |
Context of legal framework and ethical codes of conduct: Protection of personal data act [101]; Code of conduct for research and statistics [102] Age: Processing of personal data of children under 16 (if no “legitimate interest”) requires parental approval. Over 16 children themselves is enough [101] Consent: Processing of personal data, only with unambiguous consent of the data subject unless research sponsor possess “legitimate interest”. There are several codes of conduct [101] [102] Role of children: Consent of children over 12 in secondary school is sufficient Role of parents: Parental consent for children between 12 and 16 Schools: Consent of children over 12 in secondary school is sufficient. Unless a different agreement with school authorities Institutions: As above, unless a different agreement with institution authorities Ethical approval: Ethical committees can be formed in different institutions. Other Sources: [104] |
|
| Poland |
Context of legal framework and ethical codes of conduct: See sources [106], [109], [110], [111] Age: Informed consent by parents for <13 [106] Consent: Non-obligatory No regulation on the need of written informed consent but written form is preferred Role of children: for children >13 their approval is sufficient. Non-obligatory Role of parents: Informed consent for children <13. Non-obligatory Schools: Written permission form head teacher, parental consent is optional in such a protected environment (in practice parental consent is usually asked for children under 13) willingness of child to participate Institutions: n/a Ethical approval: Approval by institutional ethical committees is necessary for funding and publishing [108] Other Sources: n/a |
|
| Portugal |
Context of legal framework and ethical codes of conduct: Law of Data Protection [113] Age: n/a Consent: Individual consent for sensitive data, no specific information about children. Role of children: Voluntary participation (ibidem) Role of parents: Parental consent in a written form for children up to 18 (ibidem) Schools: Obligatory - In case of sensitive data (see below) parents’ permission beforehand Institutions: n/a Ethical approval: Obligatory formal declaration about research signed by study supervisor [118] |
|
| Romania |
Context of legal framework and ethical codes of conduct: Law No.272/2004 on the protection and promotion of the child [121]; Article 42 of the Civil Code [122]; Regulations for the operation of the Ethics Commission of the Institute of Philosophy and Psychology ”Constantin Rădulescu Motru” [123] Age: Two categories of child: <14 lack of legal capacity Consent: Obligatory:Vary from an institution to another [123] [124] [125] [126] Role of children: n/a Role of parents: n/a Schools: Example of project conducted by the ”Francisc I. Rainer” Institute for Anthropology of the Romanian Academy - Prior to starting the research, the school, the parents and the children are informed about the purpose and the methods of the research and the manner in which the collected data will be used, as well as the responsibilities and the rights of participants in the research. The participants’ oral and written consent is secured, a partnership agreement is concluded with the institution where the study is carried out, and the (progress or final) results are communicated to those involved. Institutions: Prior agreement of the local child protection services. Ethical approval: No framework code of ethics for research but many institutions adopted their own codes of ethics. Approval of the ethics committee must be sought [125] |
|
| Slovakia |
Context of legal framework and ethical codes of conduct: National Action Plan for Children 2009-2012; National Action Plan for Children 2013-2017 [129] Ethical Code of Slovak Archive of Social Data; Civil Code; ESOMAR code of ethics [130] Age: Children all up to the age of 18 [131] Consent: n/a Role of children: n/a Role of parents: Practice - Internal regulations of informed consent of parent [132] Schools: n/a Institutions: n/a Ethical approval: n/a |
|
| Slovenia |
Context of legal framework and ethical codes of conduct: Research and Development Act[138], Personal Data Protection Act[140], Patient Rights Act [141], Marriage and Family Relations Act [142], Code of Ethical Conduct and Expert Standards of Speech Therapists of Slovenia [143], Code of Professional Ethics of the Slovenian Sociological Association [144], ICC/ESOMAR International Code on Market and Social Research [146], National Medical Ethics Committee [139] , Helsinki Declaration, Oviedo Convention on Human Rights and Biomedicine and additional protocols, Slovene Code of Medical Deontology [150] Age: < 15 Parental consent Consent: Obligatory. Maybe be written, oral or in some other appropriate manner [141] Role of children: < 15: no legal capacity, if not otherwise stipulated by the law. Role of parents: <15: parental consent is mandatory Schools: Personal data of pupils may be disseminated for the purpose of scientific research work and the preparation of statistical analyses in such a manner that the pupils concerned cannot be identified [151], [152],[153] Institutions: n/a Ethical approval: The national medical ethics committee decides on the research proposals in the field of biomedicine as well as the research initiatives including people with mental health issues [139] |
|
| Spain |
Context of legal framework and ethical codes of conduct: Ley Orgánica 15/1999, de 13 de diciembre, de Protección de Datos de Carácter Personal [157] ; Code of psychologist [158] ; Law 41/2002, Royal decree 223/2004, Law 14/2007 [159] Age: n/a Consent: No regulations, only Codes of ethics of different professional organisations and international codes such as ESOMAR, ISA. Role of children: No clarity, following international codes. Code of psychologist says parents need to be informed [158] Role of parents: Practice - Parents not required consent in 2000 research from the Ombudsperson. Age groups covered were 12 to 16 years Schools: n/a Institutions: n/a Ethical approval: Universities can have their own ethical committees. Network of committees under: http://www.ub.edu/rceue/ (however it relates mostly to medical experiments and research with animals) [160] Other Sources: n/a |
|
| Sweden |
Context of legal framework and Eehical codes of conduct: Act concerning the Ethical Review of Research involving humans [161] ; Swedish Government, The Swedish Medical Products Agency; The Data Inspection Board Age: Children between 15-18 years are in need of extensive protection, beyond making sure that they undertake activities freely and with awareness of possible adverse consequences [161] Consent: Obligatory parental consent for children <15 Role of children: Children consent from 15 to 18. Assessment of the level of maturity and capacity for insight is needed [161] [163] Role of parents: For children below 15: Parents must be informed and give consent Schools: n/a Institutions: n/a Ethical approval: six regional ethics review boards (REPNs) that review research projects. Ethics review board should review a research project if any of the following conditions exist: Other Sources: [162] |
|
| UK |
Context of legal framework and ethical codes of conduct: Ethical guidelines published by the Medical Research Council (MRC) [164] , Framework for Research Ethics (FRE) published by the Economic and Social Research Council (ESRC) [170] ; Market Resarch Society (MRS) [171] ; United Kingdom (1969) Family Law Reform Act 1969, s 8(1) [166] ; United Kingdom (1991) Age of Legal Capacity (Scotland) Act 1991, s 1 (1) and s 2 (4) [168] ; United Kingdom (1995) Children (Scotland) Act 1995, s 6(1). [169] Age: Child <18 (General legal principles) Consent: <16 Parental consent Role of children: Child’s ability to give consent to medical treatment will depend on whether or not the child has achieved “sufficient understanding and intelligence to enable him or her to fully understand what is proposed". The emphasis placed on obtaining the informed consent of children wherever possible and, where not possible, at least securing their 'assent' to participation [164] [166] Role of parents: see above Schools: Good practice to seek additional consent from the child’s parents [171] Institutions: n/a Ethical approval: Researchers working with children are registered with the Independent Safeguarding Authority (ISA) and must have a clear Criminal Records Bureau (CRB). Research needs to be subject to appropriate ethics review, approval and monitoring. |
|
Austria
- Austria, Federal Law concerning the Protection of Data (Datenschutzgesetz, DSG) (2000)
- Information provided by Statistics Austria, 2012
- Austrian Society of Children and Youth Medicine (Österreichische Gesellschaft für Kinder- und Jugendheilkunde ) (2001) Ethics in pediatric (Ethik in der pädiatrischen Forschung)
- Forum of the Austrian Ethics Commissions (Forum Österreichischer Ethikkommissionen) (2002) Recommendations for information of patients – consent regarding research on minors (Empfehlungen für die Patienteninformation –Einwilligungserklärung bei Studien an Minderjährigen)
- Austria, Federal Law of 2 March on Production and Marketing of Medicinal Products (Bundesgesetz vom 2 März 1983 über die Herstellung und das Inverkehrbringen von Arzneimitteln (Arzneimittelgesetz - AMG), 1983
- Network Children’s Rights Austria (Netzwerk Kinderrechte Österreich) (2014) ‘Feedback’
Belgium
- Belgium, Law of 8 December 1992 on the protection of privacy in relation to the processing of personal data (Private Life Act), (Loi du 8 décembre 1992 relative à la protection de la vie privée à l'égard des traitements de données à caractère personnel (Loi vie privée)
- Belgium, Law of 7 May 2004 concerning experiments on humans (Loi relative aux expérimentations sur la personne humaine)
- Information provided by the Educational and Psychology Department, Vrije Universiteit Brussel, 2012
Bulgaria
- Bulgaria, Child Protection Act (Закон за закрила на детето) (2000)
- Bulgaria, Ethical Code for People Working with Children
- Bulgaria, Ethical Code of the Bulgarian Media (2004)
- Bulgaria, Personal Data Protection Act (Закон за защита на личните данни) (2002)
- Bulgaria, Family Code (Семеен кодекс) (2009)
- Bulgaria, Health Act (Закон за здравето) (2005)
Croatia
- Croatia, Council for Children (2003), Code of Ethics for Research Involving Children (Etički kodeks istraživanja s djecom)
- Croatia, Ministry of Science, Education and Sport (2008) National Pedagogical Standard for Preschool Education (Državni pedagoški standard predškolskog odgoja i naobrazbe)
- Croatia, Ministry of Science, Education and Sport (2008) National Pedagogical Standard for Elementary Education (Državni pedagoški standard osnovnoškolskog sustava odgoja i obrazovanja)
- Croatia, Ministry of Science, Education and Sport (2008) National Pedagogical Standard for Secondary Education (Državni pedagoški standard srednjoškolskog sustava odgoja i obrazovanja)
- Croatia, Act on the Protection of Patients’ Rights (Zakon o zaštiti prava pacijenata), 2004
- Croatia, Ministry of Health and Social Welfare (2007) Procedures for Clinical Research and on Good Clinical Practice (Pravilnik o kliničkim ispitivanjima i dobroj kliničkoj praksi).
Cyprus
- Cyprus National Bioethics Committee (CNBC) (2005) The Operational Guidelines (Code of Practice in biomedical research) for the Establishment of Ethics Committees in reviewing Biomedical Research involving Human Subjects (Κ.Δ.Π. 175/2005)
- Cyprus, Center of Educational Research and Evaluation (CERE) (2014) Guidelines for the submission of the online application
- Information provided by the Center for the Study of Childhood and Adolescence, Cyprus, 2012
- Cyprus Pedagogical Institute, Ministry of Education and Culture (2014)
- Information provided by the Social Welfare Services, 2012
Czech Republic
- Czech Republic, Ministry of Education, Youth and Sports (2005) Ethical Framework of Research (Eticky ramec vyzkumu)
- ESOMAR World Research (2009), ESOMAR World Research Codes & Guidelines, Interviewing Children and Young People
- Czech Republic, Enhancing Literacy Development in European Languages – Code of Ethics (ELDEL), 2014
- American Psychological Association (APA) – Code of Ethics (APA), adopted in August 2002
- Association of Agencies for Market and Opinion Research (SIMAR), 2014
- Czech Republic, The Office for Personal Data Protection (2000), Czech Data Protection Act
- Czech Republic, Czech Labour Code, Czech Employment Act (Zakon o zamestnanosti), 2004
- Czech Republic, Medical Services Act (Zakon o zdravotnich sluzbach), 2011
Denmark
- Denmark, Act no. 429 of 31 May 2000 on Processing of Personal Data (Lov nr. 429 af 31 Maj 2000 om behandling af personoplysninger)
- Denmark, Danish Social Science Research Council (2002), Guidelines of the Danish Social Science Research Council (DSSRC) (Vejledende Retningslinjer for forskningsetik I samfundvidenskaberne)
- Denmark, The National Committee on Health Research Ethics (NCHRE) (2011), Act no. 593 of 14 June 2011 on research ethical review of health research projects (Lov nr. 593 af 14. Juni 2011 om videnskabsetisk behandling af sundhedsvidenskabelige forskningsprojekter
- Denmark, The National Committee on Health Research Ethics (2011), ‘Guidelines about Notification etc. of a Biomedical Research Project to the Committee System on Biomedical Research Ethics, No 9154, 5 May 2011’, Ethical guidelines of the National Committee on Health Research (Ethics Den Nationale Videnskabsetiske Komité)
Estonia
- Estonia, Personal Data Protection Act, (Isikuandmete kaite seadus), 2007
- ICC/ESOMAR International Code on Market and Social Research, 2007
- Estonia, Medicinal Products Act (Ravimiseadus), 2005
- Statues of the Research Ethics Committee of the University of Tartu (2010)
- Statues of the Tallinn Medical Research Ethics Committee (2005)
Finland
- Finland, Personal data Act (Henkilötietolaki 523/1999/ Personuppgiftslag 523/1999), 1 June 1999
- Finland, National Advisory Board on Research Ethics TENK (Tutkimuseettinen neuvottelukunta TENK/ Forskningsetiska delegationen TENK)(2009) , The ethical principles for social science, behavioral science and humanities science research and a recommendation for organising ethical pre-evaluation (Humanistisen, yhteiskuntatieteellisen ja käyttäytymistieteellisen tutkimuksen eettiset periaatteet ja ehdotus eettisen ennakkoarvioinnin järjestämiseksi).
- Finland, Child Custody and Right of Access Act (Laki lapsen huollosta ja tapaamisoikeudesta 362/1984/ lag angående vårdnad om barn och umgängesrätt 361/1983), 1 January 1984.
- Finland, The School Board of Helsinki (2012), Instructions for research permit applicants.
France
- France, French data Protection Authority (CNIL) (2011), 'Education Guide' and the 'Higher Education and Research Guide’
- France, Public Health Code (Code de la santé publique), 2014
- France, Code of Education (Code de l’éducation), 2014
Germany
- Germany, Standing Conference of the Ministers of Education and Cultural Affairs of the Laender in the Federal Republic of Germany (Kultusministerkonferenz, KMK), 2014
- Germany, DGS German Sociological Association (Deutsche Gesellschaft für Soziologie), 2014
- Germany, DFgP German Association for Psychology (Deutsche Gesellschaft für Psychologie), 2014
- Germany, DGfE German Association for Education (Deutsche Gesellschaft für Erziehungswissenschaften), 2014
- Germany, Medicinal Products Act (Arzneimittelgesetz) (2005)
Greece
- Greece, Civil Code, 2013
- Greece, Personal Data Law, 2013
- Greece, Hellenic Data protection authority (HDPA) and self-regulatory code of advertisement and communication, 2007
- Greece, Code of Media Ethics (Presidential Decree 77/2003, OG A’ 75/28.3.2003, 2003
- Greece, Law 2619/1998 (OG A’ 132/19.6.1998), Law 3984/2011 OG A’ 150/27.6.2011
- Greece, Annual ministry circular 788/95795/T1, 2011
- Information provided by Paidopolis and HDPA , 2012
Hungary
- Hungary, Act on Informational Self-determination and Freedom of Information, 2011
- Hungary, University of Szeged, Faculty of Humanities, Institute of Psychology, Code of ethics and regulations (2011)
- Hungary, Institute of Psychology, ELTE University, "Basic Principles regarding Research and Publication"(2011)
- Hungary, National Institute of Family and Social policy, Amendments to the methodology of research with children" –(Adalékok a gyerekkutatások módszertanához – Nemzeti Család- és Szociálpolitikai Intézet, NCSSZI, Kapocs X. No 4. (51), (2001)
Ireland
- Ireland, Data Protection Acts of 1988 and 2003
- Ireland, Office of the Minister for Children and youth affairs (2010), Ethical review and children’s research in Ireland
- European Communities, Clinical Trials on Medicinal Products for Human Use, Statutory Instruments No. 190, No. 878 (2004) and No. 374 (2006)
Italy
- Italy, Civil Code (Codice civile) (1942)
- Italy, Personal Data Act (Codice in materia di protezione dei dati personali) (2003)
- Italy, National Data Protection Authority, Deontological codes attached to the Personal Data Act (2003)
Latvia
- Latvia, Protection of the rights of the Child Law (Bērnu tiesību aizsardzības likums) (1998)
- Latvia, Personal Data Protection Law (Fizisko personu datu aizsardzības likums), (2000)
- Latvia, The Latvian Sociological Association (Latvijas Sociologu asociācijas, LSA) (2008), Code of professional conduct for social and market research (Profesionālās darbības kodekss sociālo un tirgus pētījumu veikšanai)
- Information provided by the Institute of Sociological Research, Latvia
- Latvia, The Latvian Sociological Association (2008), Code of professional conduct for social and market research (Latvijas Sociologu asociācijas, profesionālās darbības kodekss sociālo un tirgus pētījumu veikšanai), 2008
- Latvia, Law on the rights of patients (Pacientu tiesību likums), 2009
Lithuania
- Lithuania, Law on the Legal protection of Personal Data, 2008
- Lithuania, Civil Code , 2000
- Information provided by Ombudsman for Children Rights (2012), National association of Childhood researchers (2012) , Psychological Innovations and Research Training Centre at Vilnius University (2012) and Novelskaitė, A. (2010) Report of a research Ethics of Scientific Research in Lithuania: Analysis of Situation. Unpublished manuscript.
- M. Mačėnaitė, D. Paulikienė, I. Skersytė, D. Šinkūnienė, Lietuvos Vartotojų Institutas (2011), Protection of Privacy of Children in the Internet (Vaikų privatumo apsauga internete), p. 69
- Lithuania, Law on ethics of biomedical research, 2007
- Information provided by the NGO Children Support Centre, 2012
- ICC/ESOMAR International Code on Market and Social Research (2007)
- Infomation provided by FRA National Liasion officer, 2013
Luxembourg
- Luxembourg, NCDP (National Commission for Data Protection) ("Commission Nationale pour la protection des données"), 2014
- Information provided by the Ministry of Education, 2012
- Luxembourg, Civil Code, 2013
- Luxembourg, Luxembourg's research fund (Fonds National de la Recherche Luxembourg)
- Luxembourg, Comité National d’Ethique et de Recherche, (2005), Grand ducal decree of 30 May 2005
- Luxembourg, The Law of 2 August 2002, on the Protection of Persons with regard to the Processing of Personal Data (Loi du 2 août 2002 relative à la protection des personnes à l’égard du traitement des données à caractère personnel)
- Luxembourg, Law of 18 March 2013 (Loi relative aux traitements de données à caractère personnel concernant les élèves)
Malta
- Malta, Data Protection Act ( 2001)
- Malta, Civil Code, Cap 16 (2014)
- Malta, Research Ethics Committee, University of Malta (UREC), Guidelines for UoM Research Ethics Committee
- Malta, University of Malta Research Ethics Committee, Guidelines (2004)
- Information provided by the Ministry of Education of Malta, Directorate for Quality and Standards in Education (DQSE), 2012
- Information provided by Children and young persons’ Advisory Board, 2012
- Foundation for Social Welfare Services (FSWS), Malta
- Malta, Processing of Personal Data (Protection of Minors) Regulations, 2004
Netherlands
- Netherlands, Protection of Personal Data Act (Wet bescherming persoonsgegevens,WBP), 2001
- Netherlands, Code of conduct for research and statistics, (Gedragscode voor Onderzoek en Statistiek), 2010
- Netherlands, Netherlands Institute of Psychologists (2007), Code of ethics of psychologists(Beroepscode voor psychologen)
- Netherlands, WMO Medical Research Involving Human Subjects Act (Wet Medisch-wetenschappelijk onderzoek met mensen, WMO)(1998)
Poland
- ESOMAR World Research (2009), ESOMAR World Research Codes & Guidelines, Interviewing Children and Young People
- Poland, Committee for Ethics in Science of the Polish Academy of Sciences, Polish Family and Guardianship Code, Civil Code (Kodeks Cywilny),1964
- Poland, Polish Psychological Association, Code of professional ethics for psychologist, (Kodeks etyczno – zawodowy psychologa)
- Institutional ethical committees of the Faculty of Psychology, Adam Mickiewicz University; Faculty of Psychology, Warsaw University; Faculty of Pedagogics and Psychology, University of Sliesia; Faculty of Psychology, Jagiellonian University,
- Poland, Office of Commissioner for the Rights of the Child (2000), Act on Commissioner for the Rights of the Child (Ustawa o Rzeczniku Praw Dziecka)
- Poland, Physician’s Profession Act (Ustawa z 5 grudnia 1996 o zawodzie lekarza),1996
- 7.Poland, Local bioethical committees, Ministry of Health Regulation of the Minister of Health on clinical research with minors (Rozporządzenie Ministra Zdrowia z dnia 30 kwietnia 2004 r. w sprawie sposobu prowadzenia badań klinicznych z udziałem małoletnich), 2004
- >Kidspeak
Portugal
- Law of Data Protection of 26 October 1998 (Lei 67/98 de 26 de Outubro)
- Information provided by the Directorate General for Education (Direcção Geral da Educação), 2012
- Portugal, Law 12/2005 of 26 January about personal genetic information, (Lei 12/2005 de 26 de Janeiro)
- Information provided by the General Director of Innovation and Curriculum Development (Direcção Geral da Educação), 2012
- Information provided by CESIS (Centro de Estudos para a Intervenção Social), 2012
- Information provided by the Directorate General for Education, 2012
- EU Kids Online Project, 2009-2011
- Information provided by Casa Pia de Lisboa, 2012
Romania
- Romania, CNECSDTI (National Council for Ethics in Scientific Research, Technological Development and Innovation), 2008
- Romania, Law No.272/2004 regarding the protection and promotion of the rights of the child (Legea nr.272/2004 privind protecţia şi promovarea drepturilor copilului
- Romania, Article 42 of the Civil Code (2011)
- Information provided by the Babeş-Bolyai University, Institute of Philosophy and Psychology “Constantin Rădulescu Motru” of the Romanian Academy, (Institutul de Filozofie şi Psihologie “Constantin Rǎdulescu-Motru”), 2012
- Information provided by ”Francisc I. Rainer” Institute for Anthropology of the Romanian Academy (Institutul de Antropologie ”Francisc I. Rainer”), 2012
- Romania, Ethics Comission, Ethic Code, University Babes-Bolyai
- Information provided by the Roma Center for Social Intervention and Studies (Romani CRISS), 2012
- Information provided by the Bucharest Municipality School Inspectorate (Inspectoratul Școlar al Municipiului București), 2012
- Romania, The General Department on Child Protection of the Ministry of Labour, Family and Social Protection (Direcţia Generalǎ Protecţia Copilului, Ministerul Muncii, Familiei şi Protecţiei Sociale) (2002), Official Gazette nr.702/2002
Slovakia
- Slovakia, Government of the Slovak republic (2009), National Action Plan for Children 2009-2012 and 2013-2017
- Slovakia, Ethical Code of Slovak Archive of Social Data 2004 – 2012
- Slovakia, Civil Code (Občiansky zákonník ), 1964
- Slovakia, Information provided by Research Institute for Child Psychology and Pathopsychology, The Slovak National Institute for Education, UNICEF, Slovak National Institute for Education, Slovak Youth Institute.
- Slovakia, Slovak Association of Research Agencies (SAVA) ‘obliges members to abide by ESOMAR Code of ethics’
- Slovakia, Healthcare Act, Act No. 576/2004 Coll. on Healthcare and Healthcare-related services.
- Slovakia, Act No 482/2002 on Personal Data Protection, 2002
- Slovakia, Act No. 305/2005 on Social-Legal Protection of the Children, 2005
- Slovakia, Act No.36/2005 on Family, 2005
Slovenia
- Slovenia, Research and Development Act (2002),(Zakon o raziskovalni in razvojni dejavnosti, ZRRD)
- National Medical Ethics Committee (2008)
- Slovenia, Personal Data Protection Act (2004a) (Zakon o varstvu osebnih podatkov, ZVOP-1)
- Slovenia, Patient Rights Act (2008) (Zakon o pacientovih pravicah, ZPacP) of 29 January 2008
- Slovenia, Marriage and Family Relations Act (1976), (Zakon o zakonski zvezi in družinskih razmerjih, ZZZDR), 4 June 1976.
- Slovenia, Code of Ethical Conduct and Expert Standards of Speech Therapists of Slovenia (1995), (Etični kodeks in strokovni standardi logopedov Slovenije), Društvo logopedov Slovenije
- Slovenia, Slovenian Sociological Association (Slovensko sociološko društvo), (1992), Code of Professional Ethics (Kodeks profesionalne etike SSD)
- Slovenia, Statistical Society of Slovenia (Deklaracija poklicne etike), Ethical code of conduct (Statistično društvo Slovenije)(1991)
- ICC/ESOMAR International Code on Market and Social Research (2007)
- Slovenia, Health Services Act, (Zakon o zdravstveni dejavnosti, ZZDej) (1992)
- Slovenia, Patient rights act, (Zakon o pacientovih pravicah, ZPacP) (2008)
- Slovenia, Ethical codes of conducts of the University of Ljubljana (Univerza v Ljubljani) (2009) and the University of Primorska (Univerza na Primorskem) (2011)
- Slovenian Code of Medical Deontology, Slovenian Code of Medical Deontology (Slovenski kodeks medicinske deontologije),Medical Chamber of Slovenia ( Zdravniška zbornica Slovenije) (1992).
- Slovenia, The Kindergarten Act (Zakon o vrtcih, ZVrt) (1996)
- >Slovenia, Elementary School Act (Zakon o osnovni šoli, ZOsn), 14 February 1996
- Slovenia, Rules on the collection and protection of personal data in music schools (Pravilnik o zbiranju in varstvu osebnih podatkov v glasbenih šolah) (2004)
- Slovenia, Mental Health Act (Zakon o duševnem zdravju, ZDZdr), (2008)
- Slovenia, Rules on the collection and protection of personal data in pre-school education, (Pravilnik o zbiranju in varstvu osebnih podatkov na področju predšolske vzgoje), (2004)
- Slovenia, the Rules on the collection and protection of personal data in pre-school education (Pravilnik o zbiranju in varstvu osebnih podatkov na področju predšolske vzgoje), 9 June 2004
Spain
- Spain, Spanish Data Protection Law (1999) (Ley Orgánica de Protección de Datos de Carácter Personal), BOE 14 December 1999
- Code of Psychologist (2010), (Código Deontológico), Consejo General de Colegios Oficiales de Psicólogos, COP (2010)
- Spain (2002), Spanish Basic Law 41/2002 of 14 November on the Autonomy of the Patient and the Rights and Obligations with regard to Clinical Information and Documentation (Ley 41/2002, de 14 de noviembre, básica reguladora de la autonomía del paciente y de derechos y obligaciones en material de información y documentación clínica); Spain (2004), Royal decree 223/2004 of 6 February 2004 regulating clinical trials using medicines (Real Decreto 223/2004, de 6 de febrero, por el que se regulan los ensayos clínicos con medicamentos); Spain (2007), Act 14/2007 of 3 July on biomedical research (Ley 14/2007, de 3 de Julio, de investigación biomédica), BOE 4 July 2007,
- Network of Ethical Committees of Universities and Public Research Centres (Red de Comités de Ética de Universidades y Organismos Públicos de Investigación, RCE)
Sweden
- Sweden, Act concerning the Ethical Review of Research Involving humans (Lag om etikprövning av forskning som avser människor), (2003)
- Sweden, Act concerning the Ethical Review of Research involving humans, (Lag om etikprövning av forskning som avser människor), 2008
- Sweden, The Swedish Medical Products Agency (Läkemedelslagen) (1992), Medicinal Products Act
United Kingdom
- United Kingdom, Medical Research Council (MRC) (2004), ‘Ethics Guide: Medical research involving children’
- United Kingdom Department of Governance arrangements for research ethics committees, A harmonised edition
- United Kingdom (1969), Family Law Reform Act 1969, s 8(1)
- United Kingdom House of Lord, Gillick v West Norfolk and Wisbech Area Health Authority, AC 112 of 17 October 1985
- United Kingdom (1991), Age of Legal Capacity (Scotland) Act
- United Kingdom (1995), Children (Scotland) Act
- Economic and Social Research Council (ESRC) (2010), Framework for research ethic (FRE)
- Market Research Society (MRS) (2012), ‘Guidelines on research with children and young people’
- Market Resarch Society, Code of Practice
- Department of Health (2005). Research Governance for Health and Social Care (2nd edition)
- United Kingdom, Medicine for Human Use (Clinical Trials) Regulations, 2004
- Ministry of Justice (27th September 2010). National Offender Management Service, Research Applications
- British Society of Criminology (2003), Code of Ethics for Researchers in the Field of Criminology, available at www.britsoccrim.org/docs/CodeofEthics.pdf; The British Psychological Society (2009), Code of Ethics and Conduct